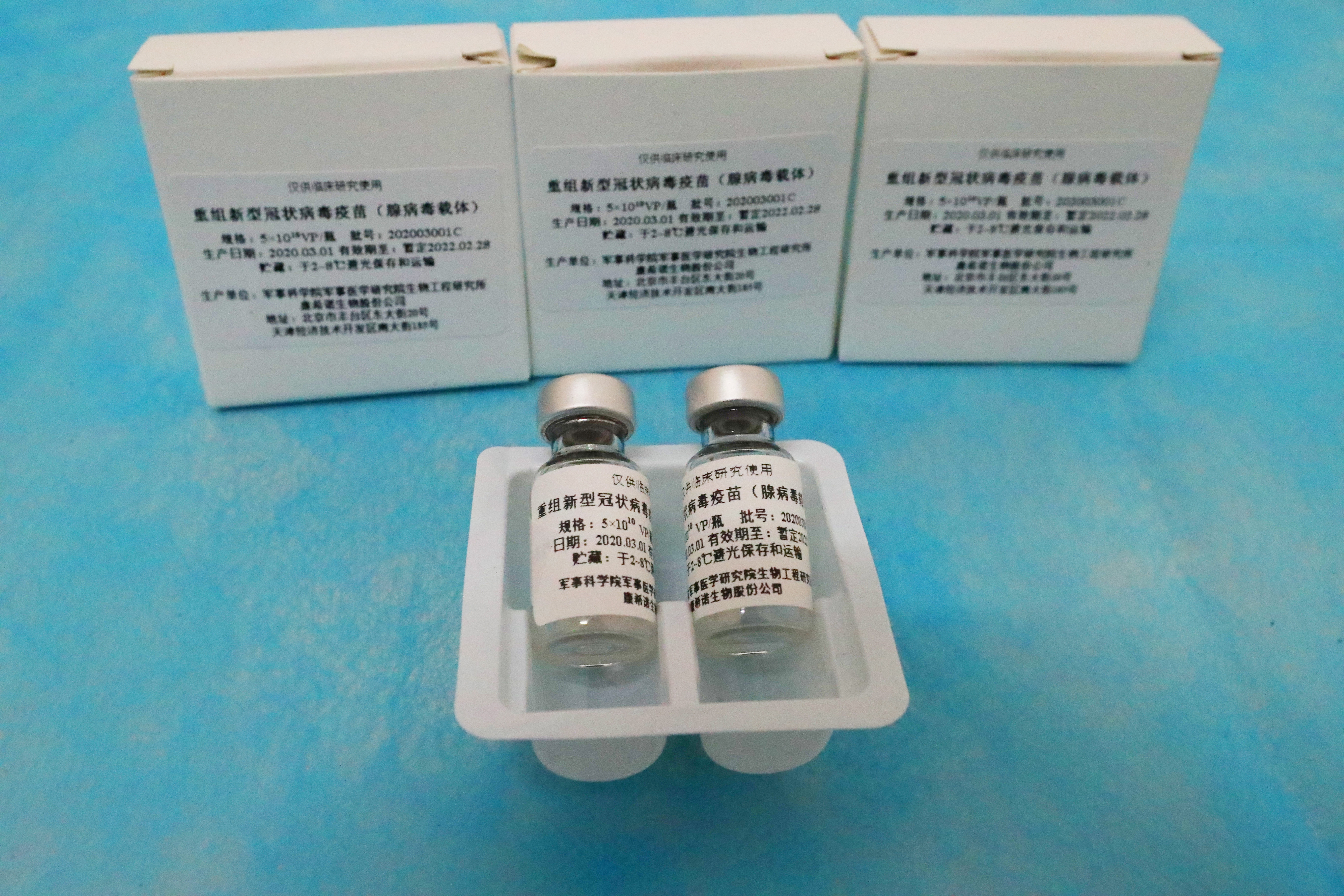
These are a part of the 35mil doses that we have ordered. The Ad5-vectored COVID-19 vaccine at 5 1010 viral particles is safe and induced significant immune responses in the majority of recipients after a single immunisation.
Single Shot Cansino Vaccine Also Stands Out For Being Easier To Store Chinadaily Com Cn
Instructions Vaccine Storage Storage and transportation in refrigerated 2-8oC condition Do not freeze the vaccine in any circumstance.
Cansino vaccine safe. CanSinos so-called viral vector vaccine which loads an antigen from the coronavirus on a harmless cold-causing pathogen called adenovirus was the first in the world to start human clinical. One-shot coronavirus vaccine by Johnson Johnson is safe and effective US FDA finds The products made by CanSino AstraZeneca Johnson and Johnson and. Delivery will be in stages.
Were just monitoring more cautiously CanSino. A coronavirus vaccine tested for the first time in humans is safe and induces a rapid immune response researchers at Chinas CanSino Biologics Inc reported on. Our findings suggest that the Ad5 vectored COVID-19 vaccine warrants further investigation.
Humoral responses against SARS-CoV-2 peaked at day 28 post-vaccination in healthy adults and rapid specific T-cell responses were noted from day 14 post-vaccination. Reuters - A vaccine against the coronavirus developed by CanSino Biologics Inc 6185HK and Chinas military research unit appears to be safe and induced immune responses in. Subsequently the vaccine had moved to.
20000 doses of the CanSino COVID-19 vaccine have arrived from China today. On May 12 the. A randomised double-blind placebo-controlled phase 2 trial - The Lancet.
Health director-general Tan Sri Dr Noor Hisham Abdullah in a Tweet said the vaccine of which its first batch landed this morning is in the form of finished product. Reuters - A coronavirus vaccine tested for the first time in humans is safe and induces a rapid immune response researchers at Chinas CanSino Biologics Inc reported on. The NRC CanSino Health Canada regulators and the CCfV agreed to set up human trials of the vaccine in Canada near the end of March 2020 Halperin said.
By Nancy Lapid Reuters - A coronavirus vaccine tested for the first time in humans is safe and induces a rapid immune response researchers at. This is also the first single. The clinical trial of the vaccine in 108 healthy adults in China showed promising results after 28 days according to the research published in prominent science journal The Lancet in May.
Is monitoring its Covid-19 vaccine more carefully after cases of blood clots forced other suppliers to suspend inoculations. It has an efficacy rate of 504 for preventing symptomatic infection according to data from a. The Ad5 vectored COVID-19 vaccine is tolerable and immunogenic at 28 days post-vaccination.
CanSino Biologics Inc. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older. The CanSino vaccine had been found to be safe and effective in generating an immune response against SARS-CoV-2 in humans in the phase 1 trials last month.
Is monitoring its Covid-19 vaccine more carefully after cases of blood clots forced other suppliers to suspend inoculations. On March 16 Chinas CanSino Biologics became the worlds first company to begin a clinical study of a vaccine designed to protect against infections of SARS-CoV-2 the novel coronavirus that causes COVID-19. Malaysia will start to administer the single-dose CanSino vaccine after receiving 200000 doses from Chinas CanSino Biologics today.
This two-dose vaccine is recommended for individuals aged 18 years and above. Each vial of CanSinos experimental COVID-19 vaccine contains 50 billion engineered adenoviruses. The plan was if demonstrated to be safe and effective by Canadian regulatory authorities for the vaccine to be manufactured in the NRCs facility in sufficient quantity to contribute to Canadas vaccine needs Halperin said.
Safe transportation storage administration and disposal of vaccine waste must be ensured at all vaccination facilities. Were just monitoring more cautiously CanSino Chairman Yu Xuefeng said on the sidelines of the Boao Forum in Hainan China. CanSino Biologics Inc.
Malaysia today received the first batch of the China-made single-dose CanSino Covid-19 vaccine which would help the National Covid-19 Immunisation Programme. CanSino vaccine needs special handling to maintain its effectiveness.

Argentina Issues Emergency Approval To China S Single Dose Cansino Covid 19 Vaccine Reuters
Malaysia S Solution Group To Supply 3 5 Mln Doses Of Cansino Vaccine To Government Reuters

Vaccine Safety And Political Legitimacy In China Council On Foreign Relations
Covid 19 Vaccines Made By China S Sinopharm Cansino Release Efficacy Data South China Morning Post
Cansinobio S Covid 19 Vaccine 65 7 Effective In Global Trials Pakistan Official Says Reuters

Pakistan Rolls Out Locally Produced Cansino Vaccine
Shepherding White Hot Global Vaccine Battle China Injects New Energy In The World S Fight Against Covid 19 Observers Global Times

Cansino Covid 19 Vaccine Shows Immune Response In Initial Stage Of Human Trial Science News

Coronavirus Confusion Over Efficacy Of Chinese Vaccines Science In Depth Reporting On Science And Technology Dw 13 04 2021

Cansino To Conduct Phase Iii Covid 19 Vaccine Trial In Saudi Arabia
Cansino Publishes First Covid 19 Vaccine Data To Muted Response

First Aerosolized Inhaled Covid 19 Vaccine Proven Safe In Phase I Clinical Trials Developers Global Times

Argentina Greenlights Emergency Use Of Cansino Vaccine
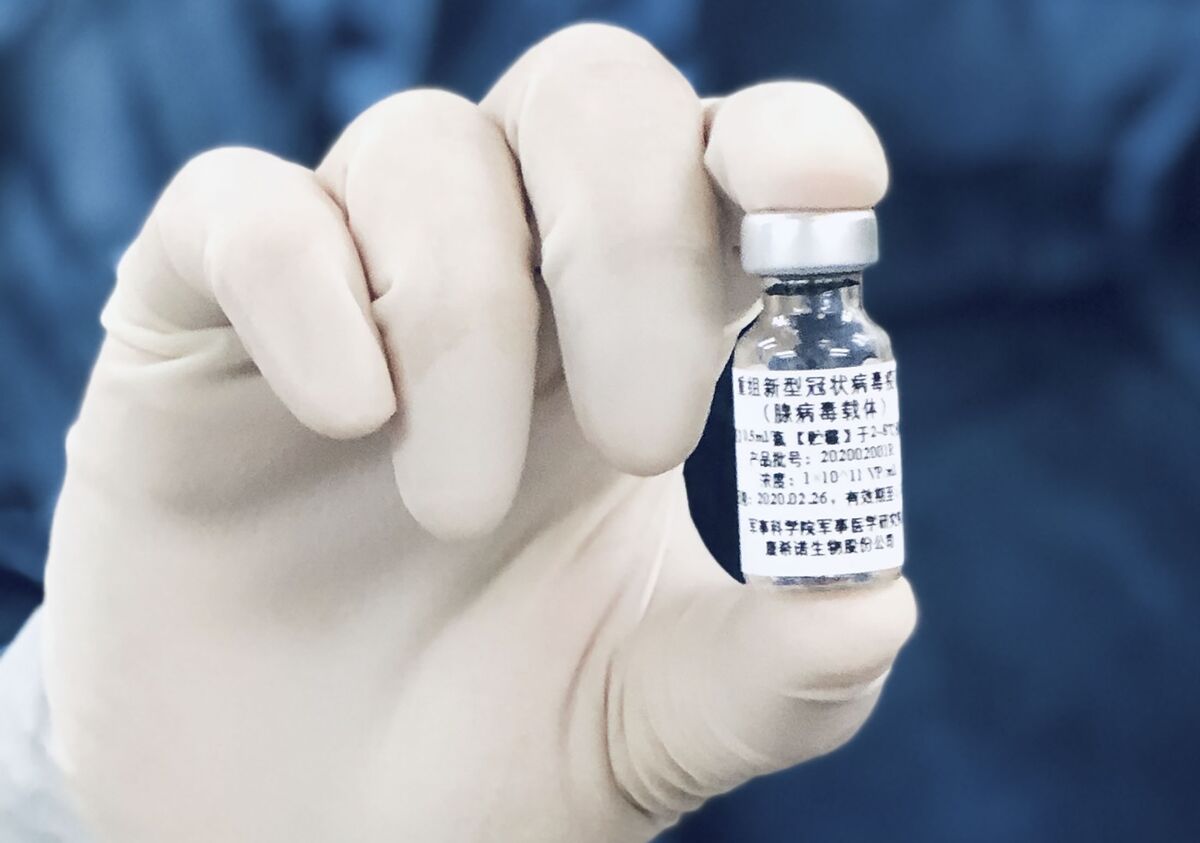
China S Cansino Vaccine Shows 65 7 Efficacy In One Shot Bloomberg

Chile Grants Eua To Cansinobio S Single Dose Covid 19 Vaccine

Cansino Bio Enrols For Ph 2 Trial Of Covid 19 Vaccine

Diplomacy Gone Amiss Story Of Chinese Covid 19 Vaccines Orf

Coronavirus Vaccine Pfizer Moderna And How Many Vaccine Doses Are Coming In 2020 Cnet

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld